MvPI (Materiovigilance Programme of India)
Materiovigilance Programme of India
The Materiovigilance Programme of India (MvPI) is an initiative taken by Central Drugs Standard Control Organization (CDSCO) in collaboration with the Indian Pharmacopoeia Commission (IPC), which operates under the Ministry of Health and Family Welfare, Government of India. The program focuses on monitoring and ensuring the safety of medical devices in the country and hence improving patient safety. NIPER Hajipur is one of the recognised centres for reporting adverse events associated with medical devices in the Bihar State to the Indian Pharmacopeia Commission, Ghaziabad (IPC).
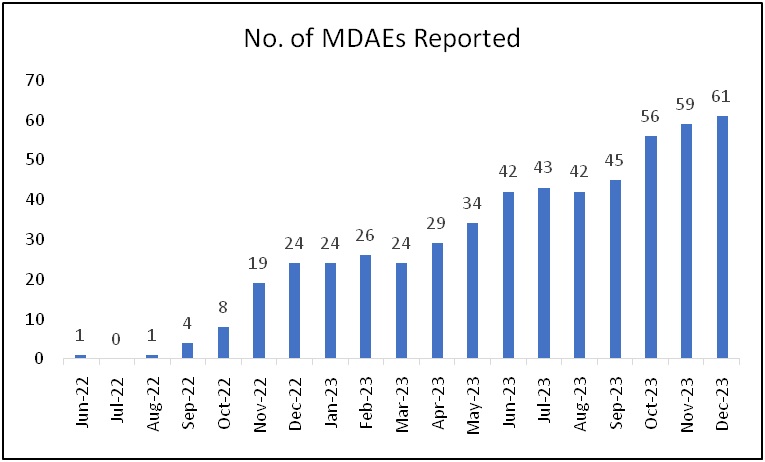
NIPER Hajipur MDMC is actively participating in this programme since June 2022 and has reported more than 500 AEs until 2023. It is also among the top-5 performing centres all over India. NIPER Hajipur MDMC is also a recognised regional training centre for educational and training programme for Materiovigilance activities to the neighboring states. NIPER Hajipur has conducted various training programmes for creating awareness on medical devices since its inception. Recently, it has also conducted a two-day training programme on adverse events reporting in collaboration with IPC Ghaziabad and other collaborative clinical facilities. This training program was attended by more than 300 attendees.
If you experience any adverse event associated with medical devices Click Here for details